Background: Acalabrutinib is a second-generation Bruton tyrosine kinase inhibitor (BTKi) approved by the Food and Drug Administration for the treatment of chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL). Data from phase 3, randomized, controlled trials of acalabrutinib, as monotherapy or in combination with obinutuzumab, in patients with treatment-naive (TN) and relapsed/refractory (R/R) CLL with long-term follow-up, including ELEVATE-TN, ELEVATE-RR, and ASCEND, have demonstrated sustained improvement vs comparators in progression-free survival and overall survival (OS) with cardiovascular (CV) and bleeding events consistent with previous reports (Sharman et al. J Clin Oncol. 2021; Byrd et al. J Clin Oncol. 2021; Ghia et al. Blood. 2022). As real-world evidence of the acalabrutinib effectiveness and safety profile is limited in CLL/SLL patients, this study aimed to describe time to next treatment (TTNT), OS, and CV and bleeding events in patients receiving first-line (1L) and later-line (2L+) acalabrutinib for the treatment of CLL/SLL in the real-world setting.
Methods: A retrospective, observational analysis was conducted utilizing the Optum Clinformatics © Data Mart claims and electronic medical record database to identify patients aged ≥18 years with TN or R/R CLL/SLL who initiated acalabrutinib in any line of therapy (LoT) on or after November 21, 2019 (FDA approval date for acalabrutinib). Patients with CLL/SLL were required to have ≥6 months of continuous enrollment in the Optum database system prior to acalabrutinib treatment initiation. Patients were followed until the earliest of last day of data availability, death, or end of study period (September 30, 2022). Treatment effectiveness was described as TTNT (time from acalabrutinib initiation to next LoT or death of any cause) and OS (time from acalabrutinib initiation to date of death from any cause or end of study period). The Kaplan-Meier (KM) method was used to estimate TTNT and OS. Patients who continued treatment at the last follow-up were censored. CV and bleeding events were assessed in all patients who initiated acalabrutinib. All outcomes were evaluated in patients who received 1L or 2L+ acalabrutinib therapy.
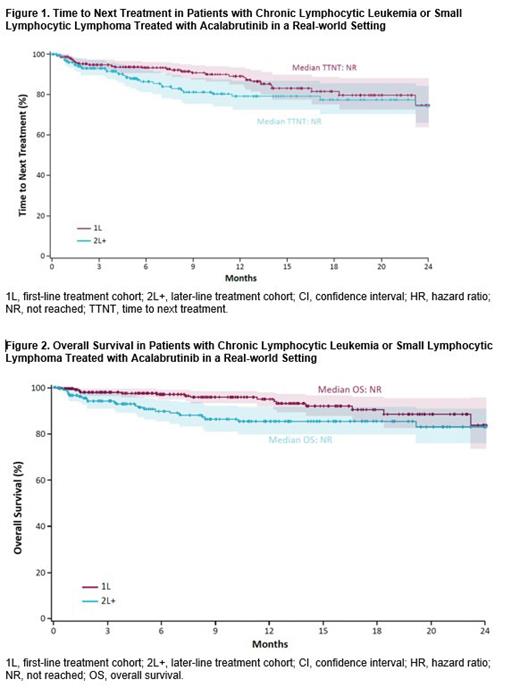
Results: A total of 530 patients were identified and included in the analysis. Of these patients, 63% (n=334) received 1L acalabrutinib and 37% (n=196) received 2L+ acalabrutinib. At acalabrutinib initiation, the mean age was 72.8 years in the 1L cohort and 74.2 years in the 2L+ cohort; 61.7% (121/196) of patients treated with 2L+ acalabrutinib had prior BTKi therapy prior to acalabrutinib initiation (ibrutinib, n=120; zanubrutinib, n=1). Distributions of gender and race were similar between the cohorts. At baseline, 18.1%, 75.3%, and 16.0% of patients had atrial fibrillation/flutter, hypertension, and bleeding episodes, respectively. With a median follow-up of 8.9 months in the 1L cohort and 12.4 months in the 2L+ cohort, the median TTNT and OS were not reached in either cohort. At 18 months, the proportion of patients remaining on treatment was 82% in the 1L cohort and 78% in the 2L+ cohort ( Figure 1). Of the patients that progressed, the median TTNT was 4.0 months in the 1L cohort (n=33) and 4.2 months in the 2L cohort (n=31). The 18-month OS rate was 90% and 85% for the 1L and 2L+ cohorts, respectively ( Figure 2). After initiation of acalabrutinib treatment, the incidence rates of atrial fibrillation/flutter, hypertension, and bleeding events were 2.7%, 8.9%, and 2.7% of patients in the 1L cohort and 7.4%, 2.4%, and 3.3% of patients in the 2L+ cohort, respectively, who had no history of these events prior to treatment with acalabrutinib.
Conclusions: This is the first study demonstrating real-world effectiveness (OS and TTNT) and safety data of acalabrutinib treatment in first-line and later-line therapy. While clinical trial and clinical practice settings may differ, overall, the survival findings in this study were similar to those of acalabrutinib trials. The observed incidence rates of CV and bleeding events were not inconsistent with those observed in published acalabrutinib trials.
Disclosures
Jacobs:Genentech: Consultancy; Adaptive: Consultancy; AbbVie: Consultancy, Research Funding, Speakers Bureau; SecuraBio: Consultancy, Speakers Bureau; Beigene: Consultancy, Speakers Bureau; Pharmacyclics: Consultancy, Research Funding, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Teneobio: Research Funding; AstraZeneca: Research Funding, Speakers Bureau; LOXO Oncology: Research Funding. Teschemaker:AstraZeneca: Current Employment, Current equity holder in publicly-traded company. Hakre:AstraZeneca: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months. Tian:Cobbs Creek Health Care LLC: Current Employment. Pyrih:Cobbs Creek Health Care LLC: Current Employment. Wahlstrom:AstraZeneca: Current Employment. Cui:Cobbs Creek Healthcare: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal